Corrado Corradi˗Dell'Acqua
Neuroscientist - Cognitive Psychologist - Data Scientist
Center for Mind/Brain Sciences - University of Trento
Biography
I am a Cognitive neuroscientist and psychologist with long-lasting experience in the study of the human adult brain, through behavioral measures, electrophysiology and brain imaging. I’m interested in the study of the neural mechanisms underlying pain, personal affect and their interaction with social cognition and decision-making.
I am also invested in understanding the cognitive and neural processes underlying the diagnosis and management of people’s pain. This is a research line with both fundamental and translational relevance, and organized on the following axes: neural/physiological fingerprinting of somatic affect, prediction of real-life pain-management behaviour from brain activity, and effect of attention on pain experience and relief.
Please check all my Research Projects
- Pain
- Chemosensation
- Affective Processing
- Social Cognition
- Decision-Making
- Cognitive Neuroscience
PhD in Neuroscience, 2007
International School for Advanced Studies (SISSA/ISAS), Trieste, Italy
MS in Psychology, 2001
Vita-Salute San Raffaele University, Milan, Italy
Skills
More than a decade of experience in the study of a human brain
Investigating brain activity with functional Magnetic Resonance Imaging
More than a decade of experience in the study of the cognitive and neural mechanisms of Pain
Proficient writer for both scientific journals (> 40 peer-reviewed articles) and funding schemes.
Extensive experience as laboratory director and team leader.
Long lasting coding experience across different programming languages (chiefly Matlab)
Extensive experience in data analysis/modeling across different platforms (R, Matlab, SPSS)
Employment of decoding techniques (SVM, Random Forest, Lasso) to predict behavior from brain activity.
If you handle two kids, you can handle anything
Experience
Grants
Research
Featured Publications
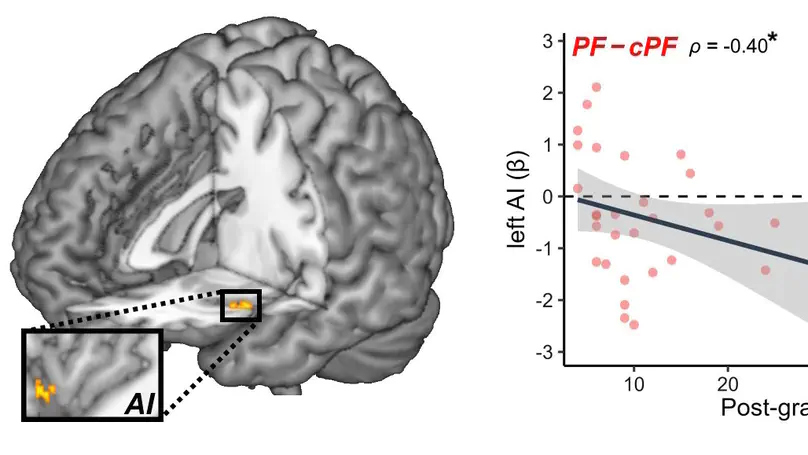
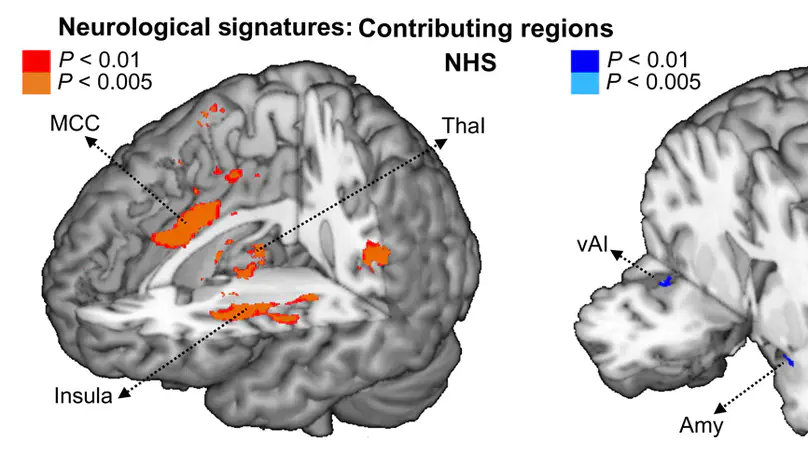
Medical students and professional healthcare providers often underestimate patients’ pain, together with decreased neural responses to pain information in the anterior insula (AI), a brain region implicated in self-pain processing and negative affect. However, the functional significance and specificity of these neural changes remains debated. Across two experiments, we recruited university medical students and emergency nurses to test the role of healthcare experience on the brain reactivity to other’s pain, emotions, and beliefs, using both pictorial and verbal cues. Brain responses to self-pain was also assessed and compared with those to observed pain. Our results confirmed that healthcare experience decreased the activity in AI in response to others’ suffering. This effect was independent from stimulus modality (pictures or texts), but specific for pain, as it did not generalize to inferences about other mental or affective states. Furthermore, representational similarity and multivariate pattern analysis revealed that healthcare experience impacted specifically a component of the neural representation of others’ pain that is shared with that of first-hand nociception, and related more to AI than to other pain-responsive regions. Taken together, our study suggests a decreased propensity to appraise others’ suffering as one’s own, associated with a reduced recruitment of pain-specific information in AI. These findings provide new insights into neural mechanisms leading to pain underestimation by caregivers in clinical settings.
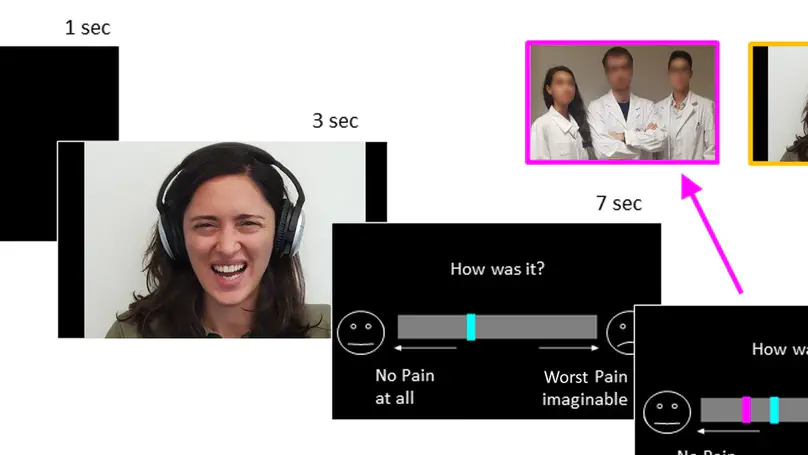
Healthcare providers often underestimate patients’ pain, sometimes even when aware of their reports. This could be the effect of experience reducing sensitivity to others pain, or distrust toward patients’ self-evaluations. Across multiple experiments (375 participants), we tested whether senior medical students differed from younger colleagues and lay controls in the way they assess people’s pain and take into consideration their feedback. We found that medical training affected the sensitivity to pain faces, an effect shown by the lower ratings and highlighted by a decrease in neural response of the insula and cingulate cortex. Instead, distrust toward the expressions’ authenticity affected the processing of feedbacks, by decreasing activity in the ventral striatum whenever patients’ self-reports matched participants’ evaluations, and by promoting strong reliance on the opinion of other doctors. Overall, our study underscores the multiple processes which might influence the evaluation of others’ pain at the early stages of medical career.
Embodied models suggest that moral judgments are strongly intertwined with first-hand somatic experiences, with some pointing to disgust, and others arguing for a role of pain/harm. Both disgust and pain are unpleasant, arousing experiences, with strong relevance for survival, but with distinctive sensory qualities and neural channels. Hence, it is unclear whether moral cognition interacts with sensory-specific properties of one somatic experience or with supramodal dimensions common to both. Across two experiments, participants evaluated ethical dilemmas and subsequently were exposed to disgusting (olfactory) or painful (thermal) stimulations of matched unpleasantness. We found that moral scenarios enhanced physiological and neural activity to subsequent disgust (but not pain), as further supported by an independently validated whole-brain signature of olfaction. This effect was mediated by activity in the posterior cingulate cortex triggered by dilemma judgments. Our results thus speak in favor of an association between moral cognition and sensory-specific properties of disgust.
